
Our expertises



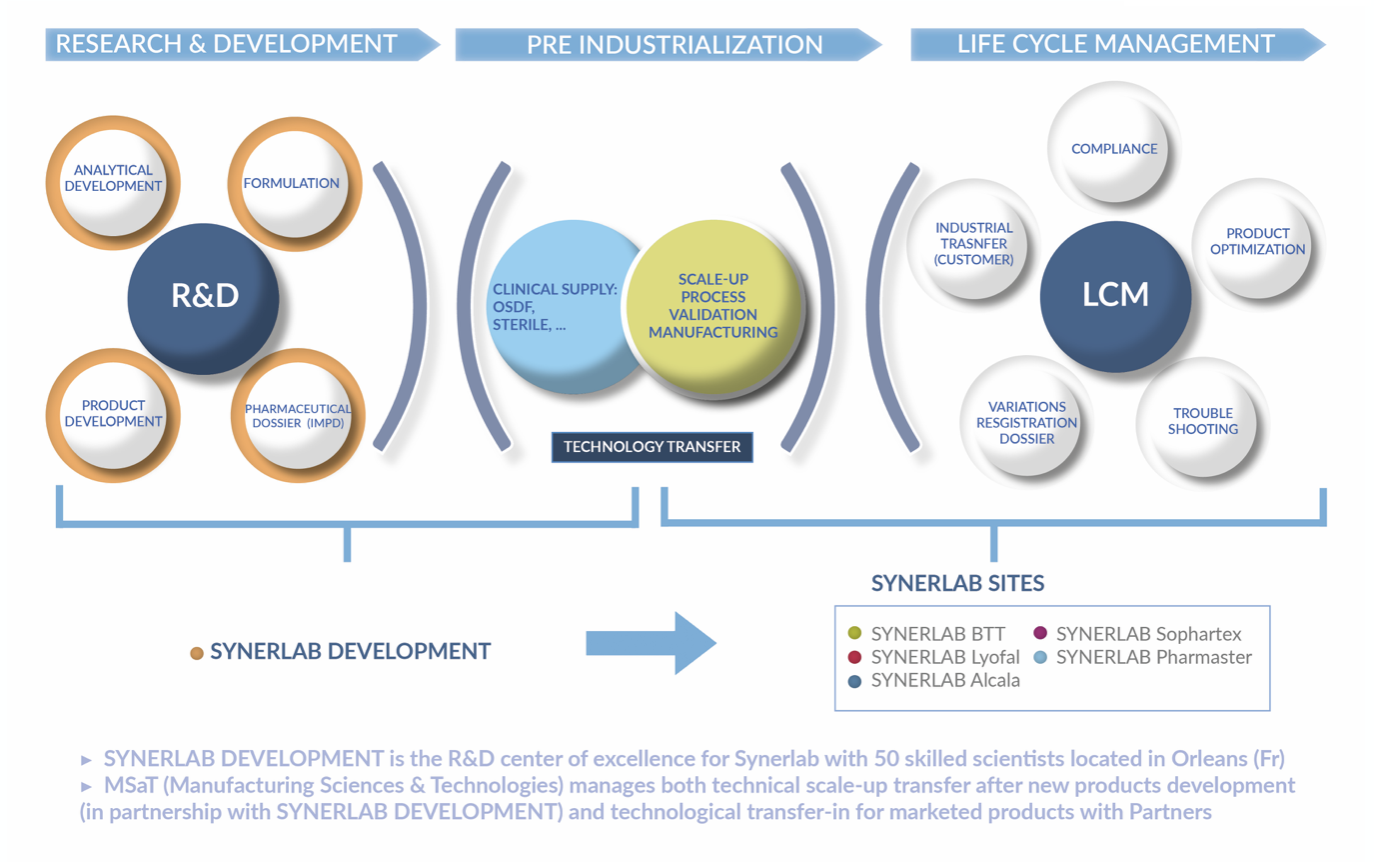
Development
As a European CDMO, Synerlab dedicate specific resources to development for their clients. Thanks to a research and development center with proven competences in drug formulation, expertise in galenics and analytics and phase 1 to 3 production of clinical batches.
Pharmaceutical development is a major business unit of Synerlab Group
Being able to bring to market new treatments is a major challenge for pharmaceutical firms. Among the different stages of pharmaceutical development, that of formulation more and more associated with specific processes requires agility and expert skillset. Some majors in the industry tend to outsource development as of the formulation stage in order to maximize their success as act faster. Thanks to long lasting client relations, a real asset in terms of pharmaceutical development, Synerlab Group can offer a complete expertise from formulation to industrialaisation, including issuing clinical batches in diverse areas (new chemical entities, OTC, freeze dried products, liquid sterile non injectables).
Thanks to a specialised development center with a proven experience in producing liquid and solid clinical batches and one in servicing analytical and regulatory requirements, completed by a unique expertise in specific niche technical processes such as freeze drying and liquid sterile non injectables, Synerlab Group is able to offer a diverse and global development set of services.
Because of this very diversity, Synerlab Group have created the right synergies for cross fertilization of savoir faires and capacity through a project based approach of different dossiers. Inspired by best practices, they offer guarantees of a robust solution servicing development demands through to industrialisation phase.

Solids
Tablets, coated tablets, dragees, capsules and soft gel capsules
Solids remain the most common form in present medical treatment as well as food supplement. Active on most common solid forms on their different plants, Synerlab Group have established a specific skillset in producing soft gel capsules, a more modern form allowing faster assimilation with a lower dosage. One of their Spanish sites in Guadalajara is entirely devoted to this type of production and is GMP certified.


Monodose
Non sterile (sachets, powder and liquid sticks)
Non sterile monodose is a fast growing form responding to pre dosage expectations as well as our nomadic lifetstyle. It allows patients to be given the right dosage of treatment in any situation of our daily life : at home, at work, when travelling… Synerlab Group offer different expertises for the packaging of this particular form whether the well known powder sachet or the more recent powder or liquid stick. SYNERLAB Group can offer remarkable production capacities, particularly for sachets of different size as well as a different positioning on small to medium series of powder or liquid sticks. Recent investment in our production chain result in more modern equipment which we ally with a rigorous approach to operational excellence. As a result, we have obtained significant production upsides as well as tighter costs in order to always improve our clients offer.

Multidose
Sterile liquid products, with or without preservatives
As a specialist in manufacturing and aseptic filling of non injectable sterile liquid products, with or without preservatives, for ENT, Synerlab is always at the forefront of this expertise in order ton anticipate market evolutions. Nowadays, it is a key player internationally in eye and ear drops, as well as nasal, topic or oral drops on a fast growing market.
Capital is the engine of this activity and Group are constantly investing in new equipments. Most recent equipments allowed to double manufacturing capacity to reach 25 million units in the next 3 years.


Freeze drying
Sterile and non sterile forms
Freeze drying appears to be the most secure and fastest technique to market biomolecular drugs. Due to undercapacity, producing freeze dried drugs requires massive investment.
Thanks to 2 sites located in France and Spain, specialised in freeze dried sterile and non sterile drugs, Synerlab recently invested 5 million euro to increase caapcity. As of 2018, the French site have been approved to produce sterile freeze dried injectable cytotoxic for human consumption and experimental ventures.
The value proposition generated through two sites specialized in freeze drying is unique, from manufacturing process optimization to producing freeze dried characterized and documented vials, allowing their clients to differentiate on their own markets.
